Titration And Neutralization Chemistry Worksheet Answers . Titration calculations & answers 1. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Use the information to determine the concentration of the hydrochloric acid. She then added a few drops of the indicator phenol red. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Find the requested quantities in the following problems: If it takes 25 ml. What is the concentration of the.
from www.numerade.com
If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. She then added a few drops of the indicator phenol red. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Titration calculations & answers 1. Find the requested quantities in the following problems: 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the.
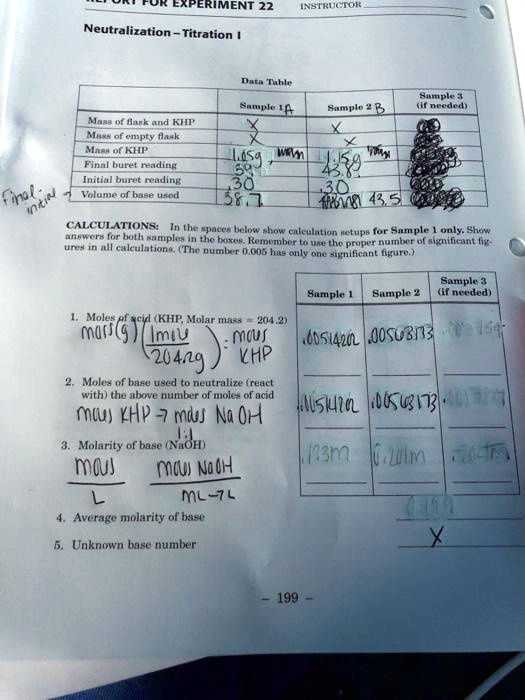
SOLVED Experiment 22 Neutralization Titration AL Tuhl Summary and
Titration And Neutralization Chemistry Worksheet Answers Use the information to determine the concentration of the hydrochloric acid. What is the concentration of the. She then added a few drops of the indicator phenol red. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Use the information to determine the concentration of the hydrochloric acid. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Titration calculations & answers 1. If it takes 25 ml. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Find the requested quantities in the following problems:
From www.numerade.com
SOLVED Experiment 22 Neutralization Titration AL Tuhl Summary and Titration And Neutralization Chemistry Worksheet Answers In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]?. Titration And Neutralization Chemistry Worksheet Answers.
From www.studocu.com
Chapter 3 acid base neutralization part C titration CHAPTER 3 ACID Titration And Neutralization Chemistry Worksheet Answers Use the information to determine the concentration of the hydrochloric acid. She then added a few drops of the indicator phenol red. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. What is the concentration of the. Find the requested quantities in the following problems: If. Titration And Neutralization Chemistry Worksheet Answers.
From www.studypool.com
SOLUTION Analytical chemistry notes on 5 principles of neutralization Titration And Neutralization Chemistry Worksheet Answers Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. She then added a few drops of the indicator phenol red. What is the concentration of the. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? In each titration, a student placed. Titration And Neutralization Chemistry Worksheet Answers.
From studyzoneburch99.z19.web.core.windows.net
Neutralization And Titration Worksheet Titration And Neutralization Chemistry Worksheet Answers What is the concentration of the. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. 1) it takes 83 ml of a 0.45 m naoh. Titration And Neutralization Chemistry Worksheet Answers.
From www.aiophotoz.com
Acid Base Titration Worksheet Answers Acid Base Titration Worksheet Titration And Neutralization Chemistry Worksheet Answers Titration calculations & answers 1. If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. What is the concentration of the. Find the requested quantities in the following problems: In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 1) 2) 3) if it takes 54 ml of. Titration And Neutralization Chemistry Worksheet Answers.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Titration And Neutralization Chemistry Worksheet Answers If it takes 25 ml. Find the requested quantities in the following problems: 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Use the information. Titration And Neutralization Chemistry Worksheet Answers.
From www.tessshebaylo.com
Chemistry Writing Equations For Neutralization Reactions Worksheet Titration And Neutralization Chemistry Worksheet Answers If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. She then added a few drops of the indicator phenol red. What is the concentration of the. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Find the requested quantities. Titration And Neutralization Chemistry Worksheet Answers.
From www.teachit.co.uk
Neutralisation worksheetKS3 chemistryTeachit Titration And Neutralization Chemistry Worksheet Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Use the information to determine the concentration of the hydrochloric acid. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? In each titration, a student placed 25.0 cm3 samples. Titration And Neutralization Chemistry Worksheet Answers.
From worksheetzone.org
Free Printable Neutralization Reactions Worksheets Titration And Neutralization Chemistry Worksheet Answers If it takes 25 ml. What is the concentration of the. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. She then added a few drops of the indicator phenol red. Use the information to determine the concentration of the hydrochloric acid. 1) 2) 3) if it takes 54 ml of 0.1 m. Titration And Neutralization Chemistry Worksheet Answers.
From materialcampuswade55.z19.web.core.windows.net
Neutralization And Titration Worksheet Titration And Neutralization Chemistry Worksheet Answers She then added a few drops of the indicator phenol red. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Find the requested quantities in the following problems: What is the concentration of the. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution.. Titration And Neutralization Chemistry Worksheet Answers.
From pdfprof.com
Reaction de neutralisation Titration And Neutralization Chemistry Worksheet Answers Titration calculations & answers 1. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Find the requested quantities in the following problems: She then added a few drops of the indicator phenol. Titration And Neutralization Chemistry Worksheet Answers.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Titration And Neutralization Chemistry Worksheet Answers Titration calculations & answers 1. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? If it takes 25 ml. 1) 2) 3) if. Titration And Neutralization Chemistry Worksheet Answers.
From www.chegg.com
Solved TITRATIONS OF NEUTRALIZATION REACTIONS DATA SHEET Titration And Neutralization Chemistry Worksheet Answers If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration. Titration And Neutralization Chemistry Worksheet Answers.
From www.worksheeto.com
11 Best Images of Acid And Base Reactions Worksheet Acids and Bases Titration And Neutralization Chemistry Worksheet Answers She then added a few drops of the indicator phenol red. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Use the information to determine the concentration of the hydrochloric. Titration And Neutralization Chemistry Worksheet Answers.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Titration And Neutralization Chemistry Worksheet Answers 1) 2) 3) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1.. Titration And Neutralization Chemistry Worksheet Answers.
From www.youtube.com
Acids and Bases, Titrations, and Neutralization Reactions YouTube Titration And Neutralization Chemistry Worksheet Answers Use the information to determine the concentration of the hydrochloric acid. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Titration calculations & answers 1. If it takes 25. Titration And Neutralization Chemistry Worksheet Answers.
From tutore.org
Worksheet Neutralization And Titration Master of Documents Titration And Neutralization Chemistry Worksheet Answers Titration calculations & answers 1. She then added a few drops of the indicator phenol red. Use the information to determine the concentration of the hydrochloric acid. In each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution,. Titration And Neutralization Chemistry Worksheet Answers.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration And Neutralization Chemistry Worksheet Answers If it takes 25 ml. Titration calculations & answers 1. Find the requested quantities in the following problems: 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? In each. Titration And Neutralization Chemistry Worksheet Answers.